Prostate cancer is the most common cancer in men
Prostate cancer is the most common cancer in men. In 2020, there were 1.4 million new prostate cancer cases worldwide, and prostate cancer-specific mortality was 370,000 men. Global prostate cancer incidence and mortality are expected to rise by 100% and 85%, respectively by 2040 as a result of the ageing population. [1]
Stockholm3 identifies more men with aggressive prostate cancer while simultaneously reducing overdiagnosis compared with PSA, and detects aggressive cancers at PSA levels as low as ≥ 1.5 ng/ml. [2]
Real world evidence from Norway, N=4,784
Real-world evidence from Sweden, N=12,405
Evidence of stage migration observed toward earlier curable cancer detection and fewer progressions to advanced and metastatic cancers

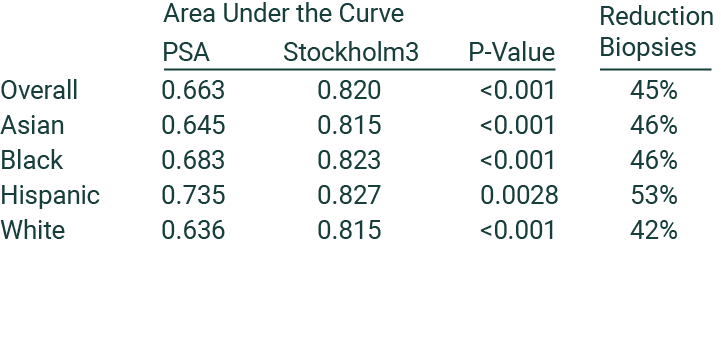
“Findings from the SEPTA trial demonstrate the utility and generalizability of Stockholm3 in reducing unneeded biopsies and detecting prostate cancer in Asian, Black, Hispanic, and White men, representing an important improvement in the harm-benefit tradeoffs in prostate cancer detection.” [3]
Iona Cheng, PhD, MPH, Professor, UCSF, Journal of Clinical Oncology
Stockholm3 is proven to work equally well in various ethnicities
The landmark SEPTA trial was carried out at 17 US and Canadian sites and is the first prostate cancer trial where most subjects were from historically underrepresented minorities. Out of 2,129 recruited men, 1,160 were Asian, Black, or Hispanic. The study found that using Stockholm3 was more accurate and could avoid up to half of the unnecessary biopsies taking place compared to current clinical practice. The findings were similar across all racial and ethnic groups. [3]
SEPTA trial: recruitment at 17 sites in the US and Canada, 2,129 biopsied men, with 1,160 minorities.
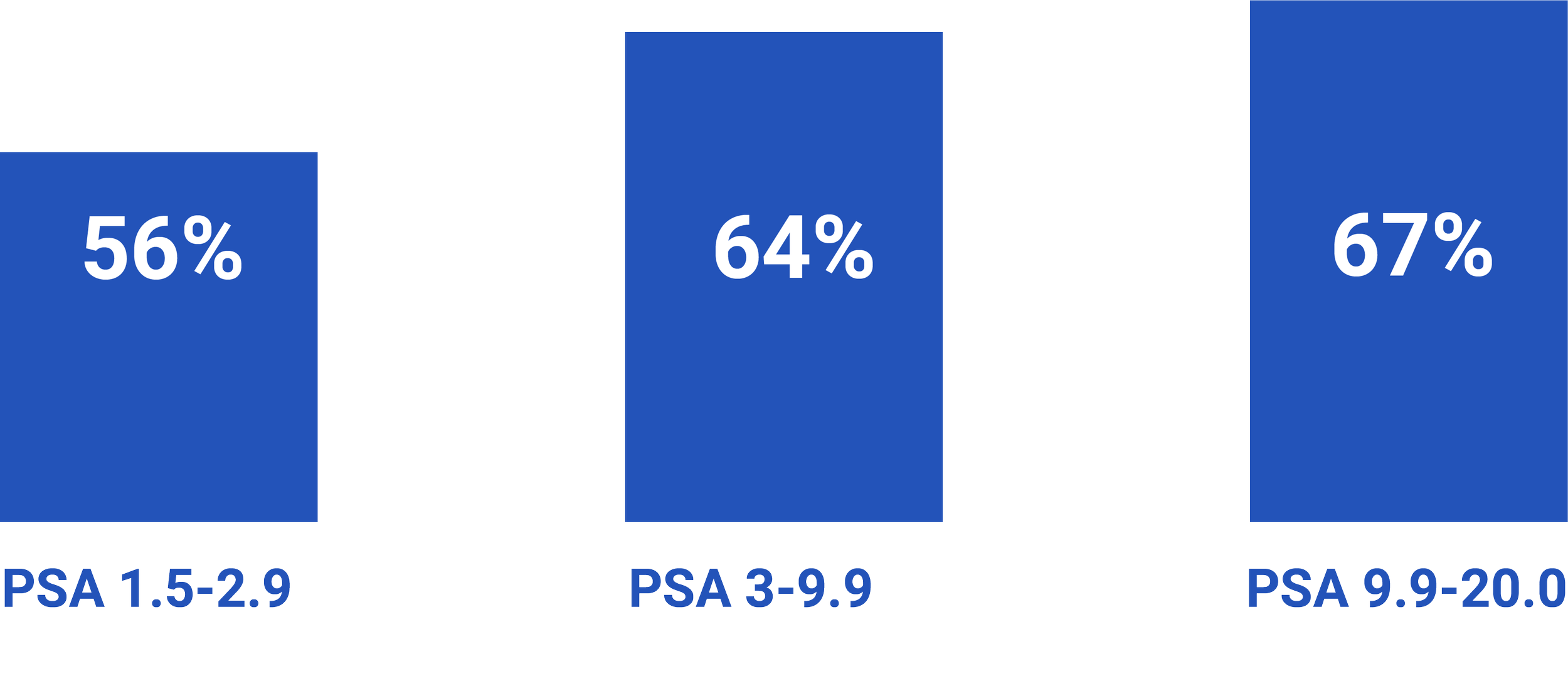
Men with elevated Stockholm3 risk score and low PSA values (1.5-2.9 ng/ml) are diagnosed with a high yield of aggressive prostate cancer at biopsy [4]
The share of ISUP ≥ 2 in biopsies after reflex testing with Stockholm3 (in PSA ≥ 1.5 ng/ml) and MRIs on positive Stockholm3.
90 000+
Participants in clinical studies [5]
41-89%
More cases of aggressive cancers found [6]
37-52%
Fewer unnecessary biopsies [7]
8-28%
Lower healthcare costs [8]
Early identification of men with aggressive prostate cancer leads to a higher than 99% survival rate.
Six reasons for Stockholm3
Stockholm3 can detect aggressive prostate cancer at an earlier stage - even in men with low PSA values (PSA values from 1.5 - 2.9 ng/ml). Early detected prostate cancer can almost always be effectively treated.
Stockholm3 can significantly reduce unnecessary biopsies and MRI examinations.
Stockholm3 has been proven to work equally well in various ethnicities, including Caucasians, African Americans, Hispanics, and Asian men.
Stockholm3 is based on clinical studies including over 90,000 European and North American men.
Stockholm3 is included in European (EAU) and American (AUA) guidelines for prostate cancer.
Stockholm3 saves significant healthcare resources and costs.[10]

“An important step towards smarter screening for prostate cancer” [11]
- Caroline M Moore, MD, Professor, University College London – Lancet Oncology Editorial
PSA: 1.5 – 20 ng/ml
Age: 40-80 years (US), 45-74 years (Europe)
No prior evidence of prostate cancer
Indications for use
-
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023 Jan
Ferlay J et al. (2024). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer
Zhao J, et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncology 2023
James et al. The Lancet Commission on prostate cancer: planning for the surge in cases. The Lancet, 2024.
2. Viste et al. Effects of replacing PSA with Stockholm3 for diagnosis of clinically significant prostate cancer in a healthcare system - the Stavanger experience. SJPHC. 2020.
Palsdottir, T et. al. The Capio Prostate Cancer Center Model for Prostate Cancer Diagnostics – Real world evidence from 2018 to 2022. European Urology Opean Science. 2024 Jan 25
3. Vigneswaran et al. Stockholm3 in a Multiethnic Cohort for Prostate Cancer Detection (SEPTA): A Prospective Multicentered Trial. J Clin Oncol. 2024 Jul 22
4. Palsdottir, T et. al. The Capio Prostate Cancer Center Model for Prostate Cancer Diagnostics – Real world evidence from 2018 to 2022. European Urology Opean Science. 2024 Jan 255. Grönberg et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. The Lancet Oncology. 2015
Nordström et al. Prostate Cancer Screening Using a Combination of Risk-Prediction, MRI, and Targeted Prostate Biopsies. The Lancet Oncology. 2021
Grönberg et al. Prostate Cancer Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic Resonance Imaging. European Urology. 2018
Viste et al. Effects of replacing PSA with Stockholm3 for diagnosis of clinically significant prostate cancer in a healthcare system - the Stavanger experience. SJPHC. 2020
Palsdottir, T et. al. The Capio Prostate Cancer Center Model for Prostate Cancer Diagnostics – Real world evidence from 2018 to 2022. European Urology Opean Science. 2024 Jan 25
Elyan et al. Prospective Multicenter Validation of the Stockholm3 Test in a Central European Cohort. Eur Urol Focus. 2023 Oct 7
Fredsoe et al. Results from the PRIMA Trial: Comparison of the STHLM3 Test and Prostate-specific Antigen in General Practice for Detection of Prostate Cancer in a Biopsy-naïve Population. Eur Urol Oncol. 2023 Oct
Vigneswaran et al. Stockholm3 in a Multiethnic Cohort for Prostate Cancer Detection (SEPTA): A Prospective Multicentered Trial. J Clin Oncol. 2024 Jul 22
Tilki et al. External Validation of Stockholm3 in a Retrospective German Clinical Cohort. Eur Urol Focus. 2024 Aug 6
6. Viste et al. Effects of replacing PSA with Stockholm3 for diagnosis of clinically significant prostate cancer in a healthcare system - the Stavanger experience. SJPHC. 2020Palsdottir, T et. al. The Capio Prostate Cancer Center Model for Prostate Cancer Diagnostics – Real world evidence from 2018 to 2022. European Urology Opean Science. 2024 Jan 25
7. Grönberg et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. The Lancet Oncology. 2015
Eklund et al. The Stockholm-3 (STHLM3) Model can Improve Prostate Cancer Diagnostics in Men Aged 50–69 yr Compared with Current Prostate Cancer Testing. European Urology Focus. 2016
Grönberg et al. Prostate Cancer Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic Resonance Imaging. European Urology. 2018
Waldén M et al. A Head-to-head Comparison of Prostate Cancer Diagnostic Strategies Using the Stockholm3 Test, Magnetic Resonance Imaging, and Swedish National Guidelines: Results from a Prospective Population-based Screening Study. European Urology Open Science. 2022
Vigneswaran et al. Stockholm3 validation in a multi-ethnic cohort for prostate cancer (SEPTA) detection: A multicentered, prospective trial. JCO supplements, ASCO-GU 2024 presentation, 2024, Jan 25. Vigneswaran et al. Stockholm3 in a Multiethnic Cohort for Prostate Cancer Detection (SEPTA): A Prospective Multicentered Trial. J Clin Oncol. 2024 Jul 22
Tilki et al. External Validation of Stockholm3 in a Retrospective German Clinical Cohort. Eur Urol Focus. 2024 Aug 6
8. Bergman et al. Structured care for men who want to get tested for prostate cancer – findings from Capio Prostate Cancer Center. Läkartidningen. 2018;115:FCDTViste et al. Effects of replacing PSA with Stockholm3 for diagnosis of clinically significant prostate cancer in a healthcare system - the Stavanger experience. SJPHC. 2020, Söderbäck et al. Improved prostate cancer diagnostics with a structured pathway including Stockholm 3 test, MRI and targeted perineal biopsies. Läkartidningen. 2023
Mcleod et al. Cost Effectiveness of Prostate Cancer Testing incorporating Stockholm3 and MRI versus standard of care. EAU 2024, April 7
Hao S et al. Cost-effectiveness of Stockholm3 test and magnetic resonance imaging in prostate cancer screening: a microsimulation study. European Urology. 2022.
9. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023 Jan;73(1):17-48. doi: 10.3322/caac.21763. PMID: 36633525, Cancer Facts and Figures, American Cancer Society, 2023
10. Grönberg et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. The Lancet Oncology. 2015
Nordström et al. Prostate Cancer Screening Using a Combination of Risk-Prediction, MRI, and Targeted Prostate Biopsies. The Lancet Oncology. 2021
Grönberg et al. Prostate Cancer Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic Resonance Imaging. European Urology. 2018
Viste et al. Effects of replacing PSA with Stockholm3 for diagnosis of clinically significant prostate cancer in a healthcare system - the Stavanger experience. SJPHC. 2020
Palsdottir, T et. al. The Capio Prostate Cancer Center Model for Prostate Cancer Diagnostics – Real world evidence from 2018 to 2022. European Urology Opean Science. 2024 Jan 25
Elyan et al. Prospective Multicenter Validation of the Stockholm3 Test in a Central European Cohort. Eur Urol Focus. 2023 Oct 7
Fredsoe et al. Results from the PRIMA Trial: Comparison of the STHLM3 Test and Prostate-specific Antigen in General Practice for Detection of Prostate Cancer in a Biopsy-naïve Population. Eur Urol Oncol. 2023 Oct
Vigneswaran et al. Stockholm3 validation in a multi-ethnic cohort for prostate cancer (SEPTA) detection: A multicentered, prospective trial. JCO supplements, ASCO-GU 2024 presentation, 2024, Jan 25. Vigneswaran et al. Stockholm3 in a Multiethnic Cohort for Prostate Cancer Detection (SEPTA): A Prospective Multicentered Trial. J Clin Oncol. 2024 Jul 22
Tilki et al. External Validation of Stockholm3 in a Retrospective German Clinical Cohort. Eur Urol Focus. 2024 Aug 6
Waldén M et al. A Head-to-head Comparison of Prostate Cancer Diagnostic Strategies Using the Stockholm3 Test, Magnetic Resonance Imaging, and Swedish National Guidelines: Results from a Prospective Population-based Screening Study. European Urology Open Science. 2022
Söderbäck et al. Improved prostate cancer diagnostics with a structured pathway including Stockholm 3 test, MRI and targeted perineal biopsies. Läkartidningen. 2023
Mcleod et al. Cost Effectiveness of Prostate Cancer Testing incorporating Stockholm3 and MRI versus standard of care. EAU 2024, April 7
Hao S et al. Cost-effectiveness of Stockholm3 test and magnetic resonance imaging in prostate cancer screening: a microsimulation study. European Urology. 2022
Mottet et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021
Wei, J.T., et al., Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J Urol, 2023.
11. Moore CM. An important step towards smarter screening for prostate cancer. Lancet Oncol. 2021 Sep;22